Abstract
Introduction
Haploidentical hematopoietic stem cell transplantation (haplo-SCT) has become an acceptable approach for many patients; however, the suitability of this procedure and outcomes regarding efficacy and safety remain unclear. Here we present a single-center experience using a conditioning regimen based on cyclophosphamide and fludarabine under outpatient conditions.
Methods:
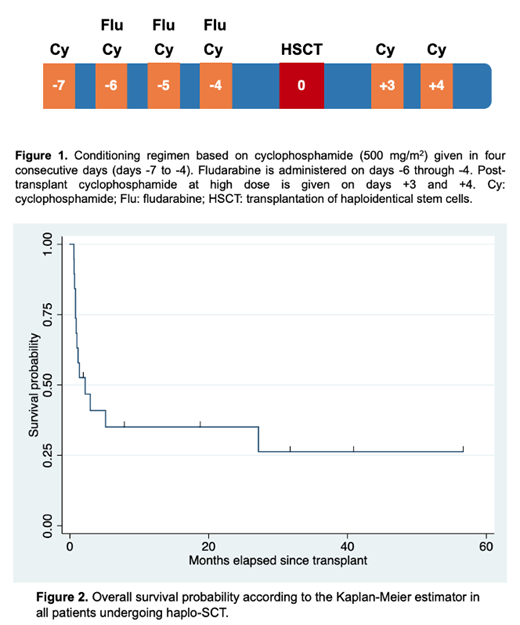
Retrospective study evaluating the performance of haplo-SCT in a single center. The conditioning regimen was fludarabine 50 mg on days -6 through -4, cyclophosphamide (Cy) 500 mg/m 2 on days -7 to -4 and tacrolimus 1000 mg and post-transplant Cy (50 mg/kg) on days +3 and +4 (Figure 1). Donor cell collection was accomplished through apheresis in all patients and fresh cells were administered on same day of collection. All the procedures were started on an outpatient basis. Overall survival was defined as mortality for any reason, starting to count from the day of transplantation to the date of death. The survival function was calculated according to the Kaplan-Meier estimation method. All patients signed a consent to participate after a thorough interview and the study protocol was approved by Clínica Ruiz IRB.
Results:
We grafted 20 patients, (14 adults and 6 children) with haploidentical cells and found that in 11 cases (55%), the full procedure could be completed totally as outpatients; the diagnosis of the grafted patients were: 10 acute lymphoblastic leukemia, 3 acute myelogenous leukemia, 3 paroxysmal nocturnal hemoglobinuria, and one each non-Hodgkin´s lymphoma, Blackfan-Diamond syndrome and multiple myeloma. Nine patients (3 children and 6 adults) were admitted to the hospital after completing the conditioning, 1 to 8 days after day 0: the causes for admission were neutropenic fever (5 cases), cytokine-release syndrome (3 cases), and intraabdominal abscess (1 case). Patients remained in the hospital for a median of 9 days. Four patients failed to engraft and recovered endogenous hematopoiesis and acute graft versus host-disease developed in 5 of 16 engrafted patients; two patients relapsed after the haplo-SCT. The transplant-related mortality was 35%, whereas the 2-year overall survival (OS) was 37.5% (Figure 2); the causes of the 7 deaths were: Four granulocytopenic sepsis, 2 graft-versus-host disease and one multiple organ failure.
Conclusions
Haplo-SCT procedures can be conducted safely and effectively on an outpatient basis; however, questions remain regarding the selection of patients and managing of complications, especially in outpatient conditions in which full and timely availability of specialized care could be the pivotal factor to improve short-term outcomes.
No relevant conflicts of interest to declare.